Put Our Proven Engineering Experience to Work for You
Innovative Process Technologies for Ammonia
Ammonia (NH3) is an essential chemical used to make fertilizers, nitric acid and urea. Leveraging the Linde Ammonia Concept (LAC™), our NH3 plants support capacities ranging from 100 metric tons per day (mtpd) to over 1,350 mtpd.
This leading-edge process produces ammonia from various feedstocks, including natural gas, light hydrocarbons and heavy hydrocarbons. The LAC process minimizes the accumulation of inert residual impurities such as argon (Ar) and methane (CH4) in the ammonia reactor, thus avoiding the need to purge syngas or invest in purge gas separation equipment. LAC benefits relative to other ammonia synthesis setups include CAPEX and OPEX savings as well as simplified plant start-up and operation.
Going beyond the LAC, Linde offers end-to-end solutions for complete ammonia facilities i.e., from hydrogen and nitrogen production to ammonia synthesis via the LAC to ammonia storage solutions. Our clients benefit from our full spectrum solutions covering all required technologies and EPC service elements for the execution of Ammonia production & storage facilities.
Low-Carbon NH3 from Light Feedstocks
The LAC concept can be applied in both conventional and low-carbon NH3 plants. Low-carbon plants help to decarbonize operations by capturing and storing or reusing carbon emissions.
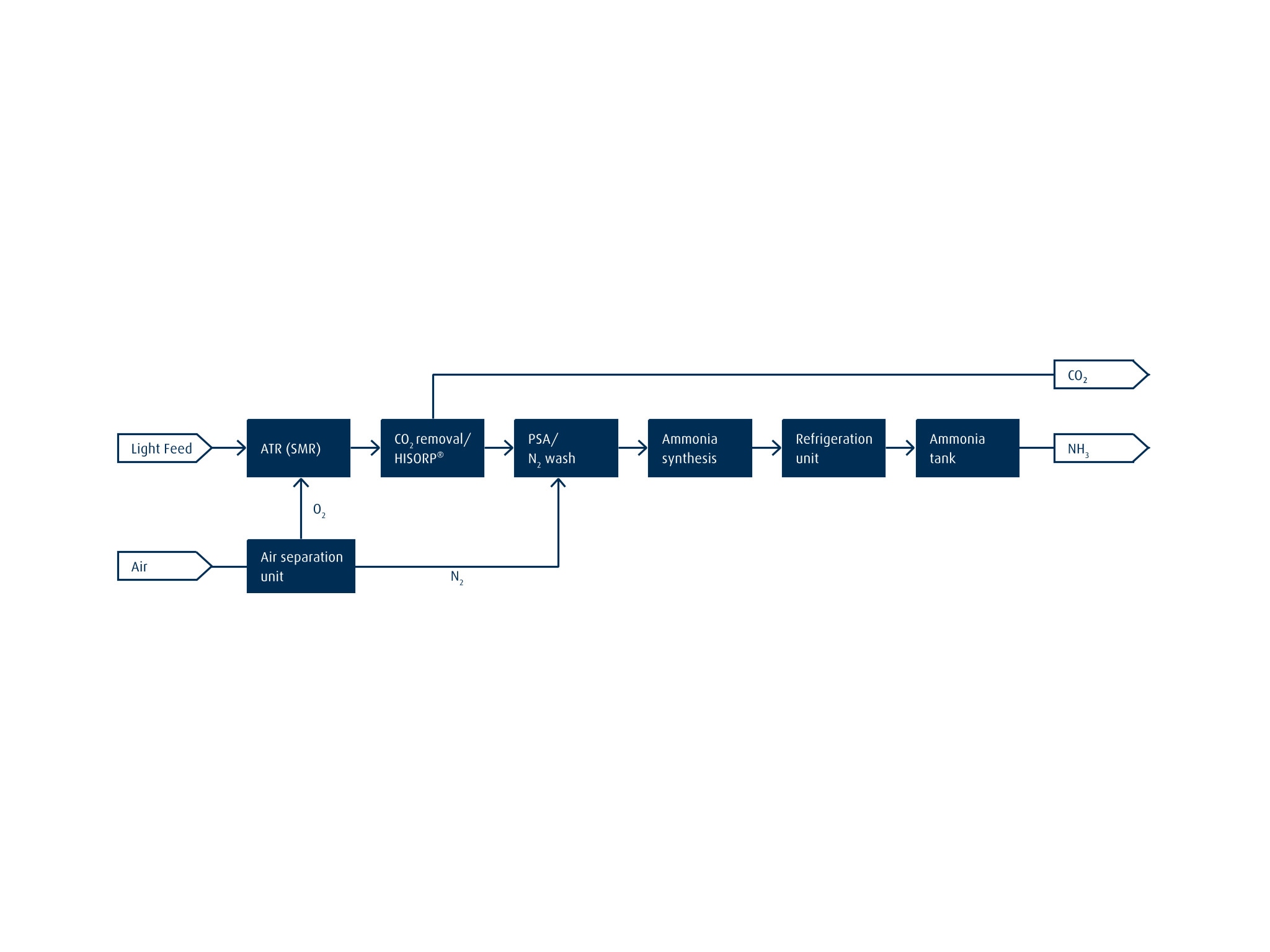
The flow chart below illustrates a low-carbon NH3 plant setup using a light feedstock. H2 is produced by means of autothermal reforming or steam methane reforming. The carbon dioxide (CO2) generated in the process is removed with a downstream HISORP CC® carbon capture solution. Depending on the feedstock used and level of impurities in the H2 product, it can be further purified by means of pressure swing adsorption or liquid nitrogen washing. Synthesis gas (syngas) with a 3:1 H2 to N2 ratio is then fed to the NH3 synthesis step. Once the produced NH3 has been cooled, it is stored in liquid form in large tanks. Learn more here about our Ammonia Storage Solutions.


Low-carbon NH3 from Heavier Feedstocks
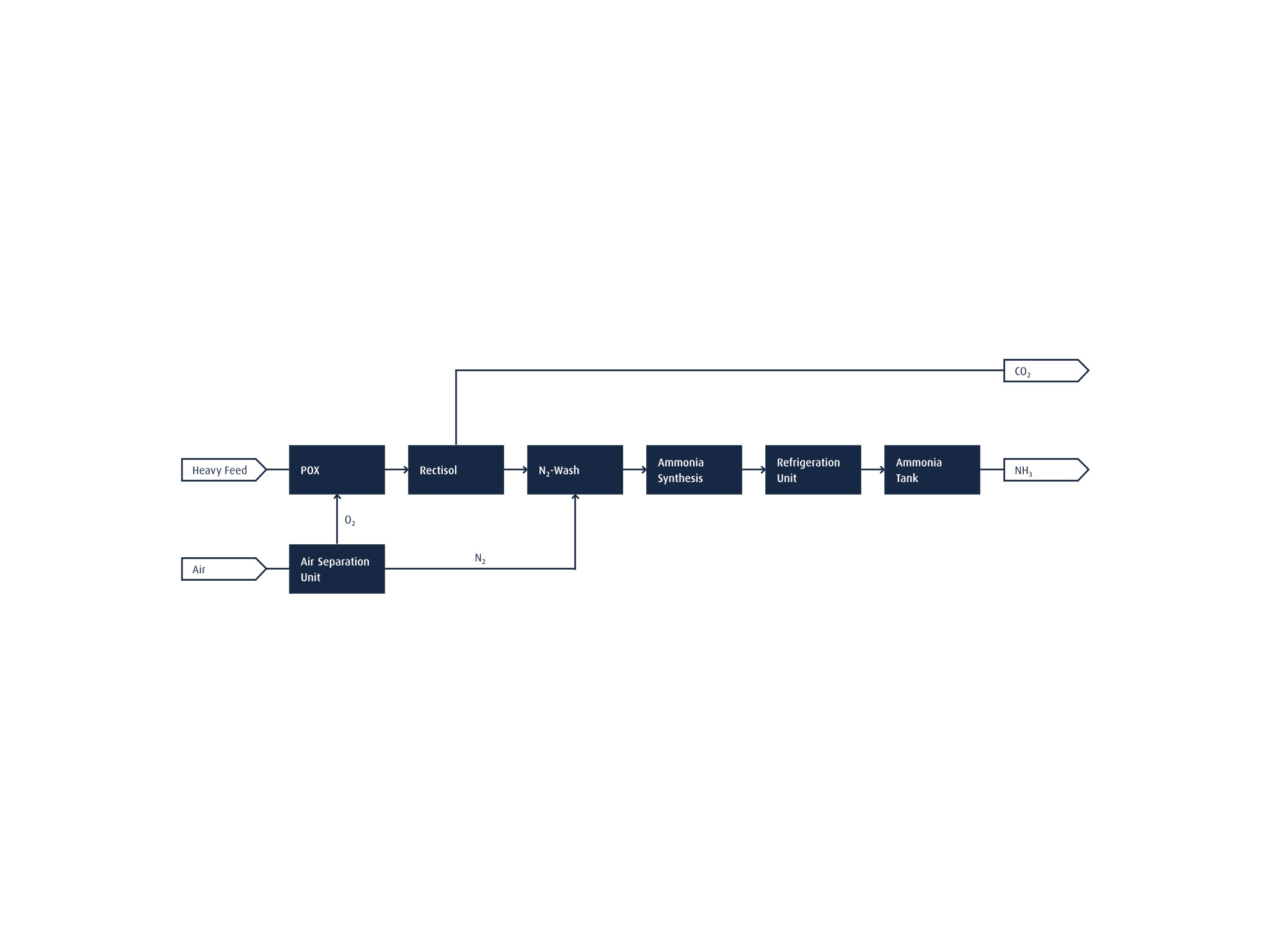
NH3 can also be produced from heavier feedstocks. Partial oxidation is used in this case, as illustrated in the flow chart to the side. The O2 required to oxidize the heavy feedstock is supplied from an on-site Linde air separation unit (ASU). A downstream Rectisol wash unit purifies the resulting syngas, removing possible sulfur contaminants and CO2. In a separate step, the CO2 is captured, also making the process "low-carbon".
This low-carbon hydrogen is then fed together with high-pressure N2 from the ASU into a liquid nitrogen wash unit. Residual impurities, such as carbon monoxide Ar and CH4, are removed and a stochiometric H2/N2 ratio of 3:1 is established for the following NH3 synthesis step.

Mega Project for H2, CO & NH3 at Sadara

| Location: | Al Jubail, Saudi Arabia |
| Focus: | HyCO + NH3 plant for the Sadara Chemical Complex |
| Scope |
Production of H2, CO and NH3, installation of NH3 tank |
| Start-up: |
2016 |
Driving the Commercialization of Renewable NH3
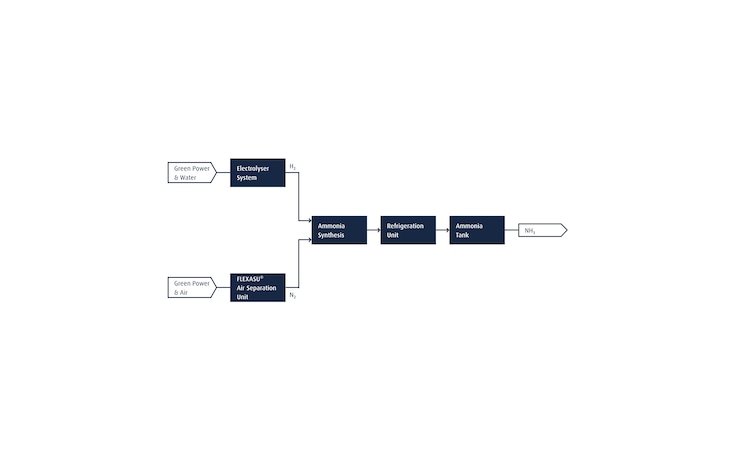
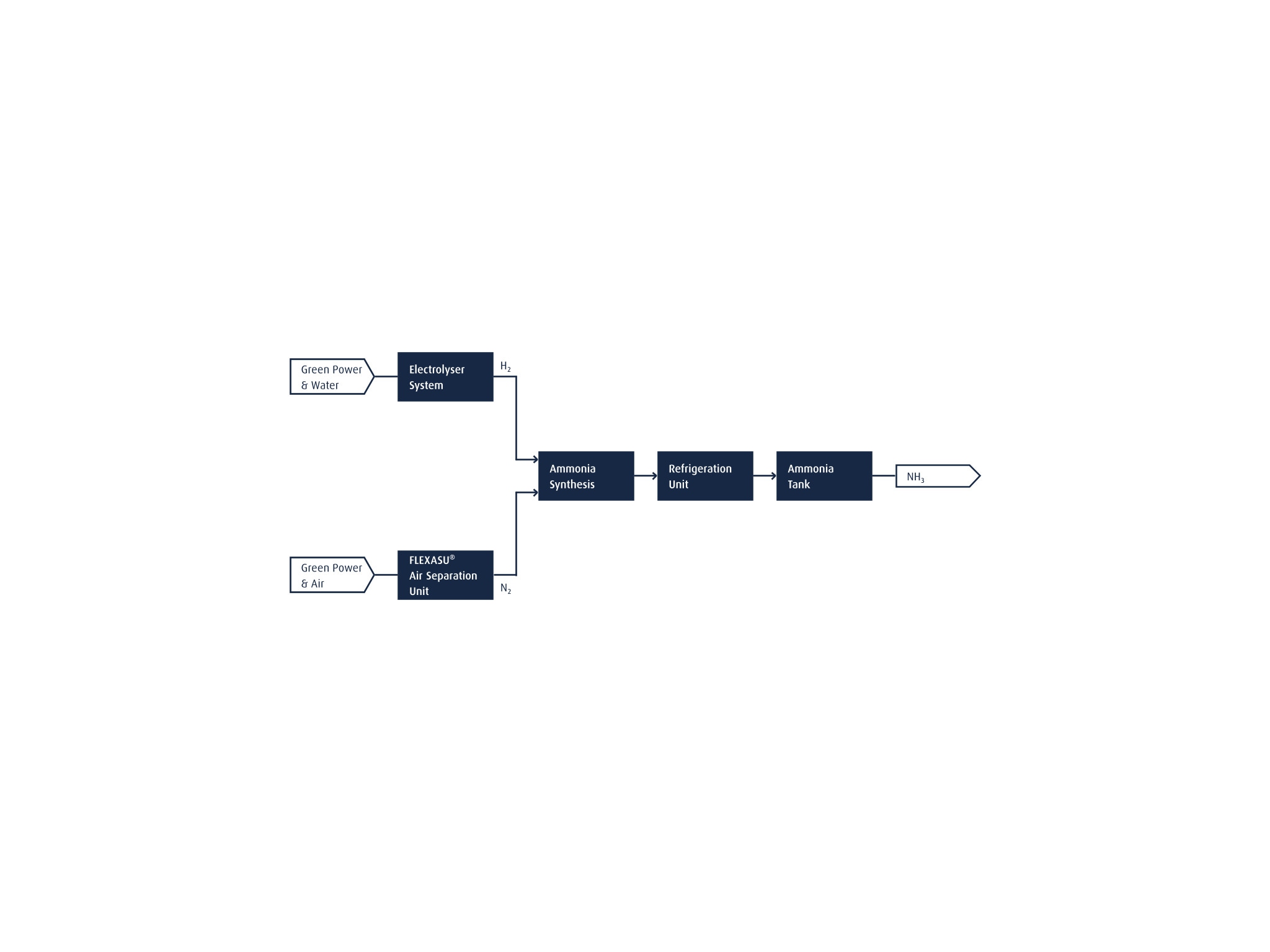
We also help our customers to further increase their sustainability performance by transitioning to renewable NH3. The flow chart below shows how a renewable NH3 plant can be efficiently realized. An electrolyzer system generates renewable H2 from water, using electricity from renewable sources. The N2 required for the NH3 synthesis step is supplied from a Linde FLEXASU® air separation unit, which can run on renewable power and supports dynamic (on-demand) operation. A H2/N2 mixture with a 3:1 ratio is then fed to NH3 synthesis step, after which the NH3 is stored in large tanks.
Yara to Demonstrate Renewable Ammonia for Fertilizer Production

| Location: | Porsgrunn, Norway |
| Focus: | Reduce annual CO2 emissions by 41,000 tons by partially replacing conventional H2 in Yara's ammonia plant with renewable H2 |
| Scope |
Build 24 MW proton exchange membrane (PEM) electrolyzer plant, which uses renewable energy to produce H2 from water for the production of renewable ammonia |
| Start-up: |
2024 |
Increasing the Reach of Methanol as an Eco-friendly Fuel
Methanol (CH3OH, MeOH) is a solvent and base chemical for various products in the chemical industry. It is also rapidly gaining in importance as an eco-friendly fuel.
It is derived from synthesis gas, which is typically produced by reforming hydrocarbons. Our MeOH technology also supports the production of renewable MeOH, where syngas is produced through biomass gasification, and e-methanol, which is created by mixing green hydrogen with imported CO2. Our advanced, proprietary isothermal reactor technology efficiently converts the syngas to high-quality MeOH.
To support individual production needs, we offer a full range of technologies extending from various syngas production methods to the actual MeOH synthesis step, complementing these with storage options for the produced MeOH and the general system integration. Building on the vast engineering and operational experience we have gained over several decades, we deliver MeOH plants with capacities ranging from 100 mtpd to over 1,000 mtpd.
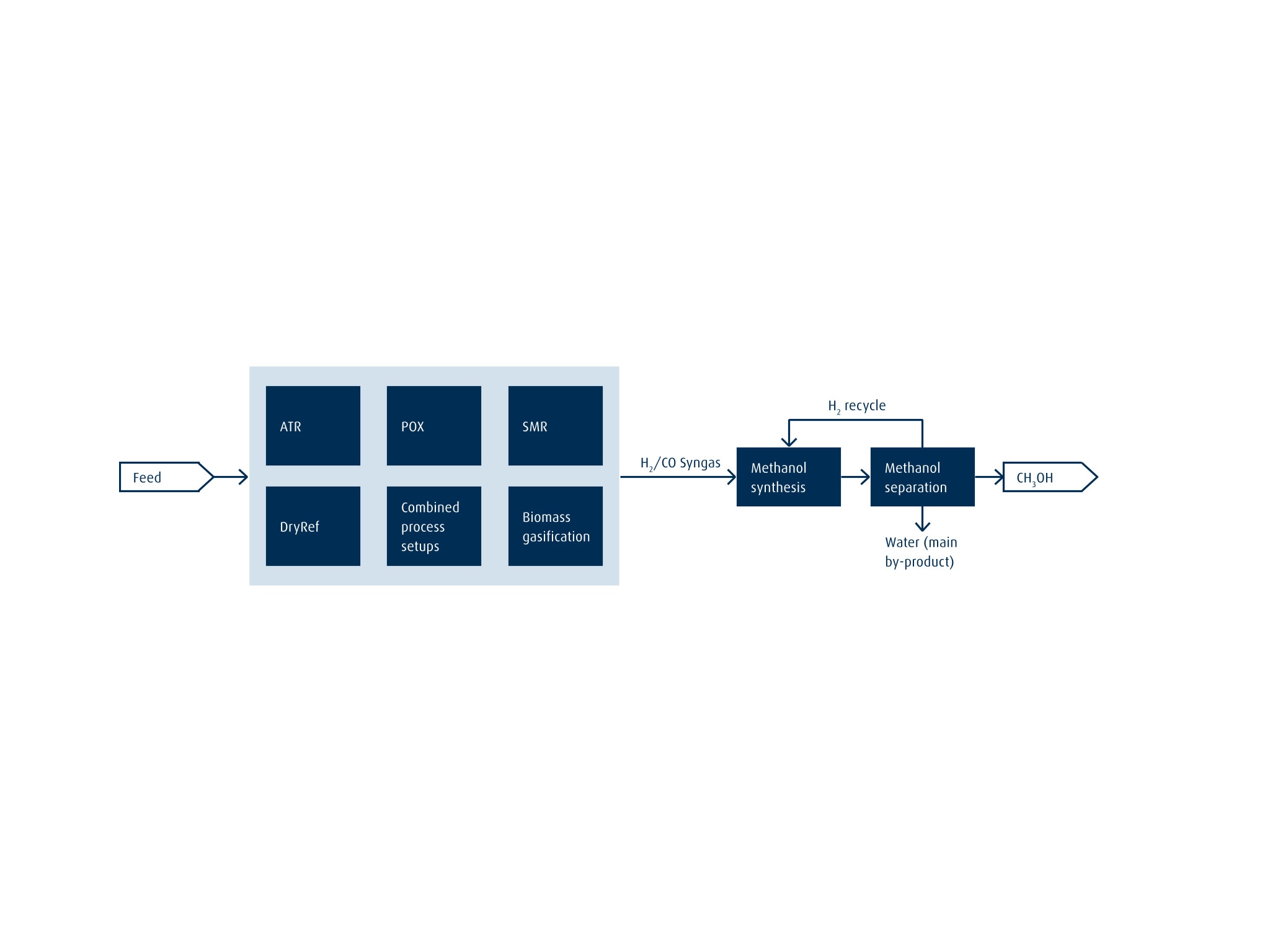
Full Spectrum from Conventional to Low-carbon
As the flow chart below illustrates, the syngas is usually generated from a hydrocarbon or biomass feedstock. Steam reforming, partial oxidation, autothermal reforming, dry reforming or a combination of these processes is used to generate the syngas. The resulting H2/CO mixture is then fed into an isothermal reactor, where the MeOH synthesis step is designed for high yield conversion rates and efficiency. In the final step, the MeOH is separated from by-products in a distillation process. Residual H2 is recirculated back to the MeOH reactor to enhance the conversion rate. The main by-product is water.
As we support all of the enabling technologies, we can tailor the process concept to each client's specific boundary conditions, balancing factors such as feedstock availability, pricing and plant size. In addition, our proven experience and extensive portfolio of CO2 capture and purification technologies means we can complement the methanol synthesis feedstock with CO2 import for MeOH with a lower carbon intensity. And if biomass is used as the syngas feed, the resulting MeOH is green, enabling customers to reduce their carbon footprint further.

CO2 and Clean H2 for Low-carbon Methanol at Celanese

| Location: | Clear Lake, Texas, US |
| Focus: | Active support for Celanese's decarbonization goals by enabling methanol with a lower carbon intensity |
| Scope: | Supply of CO2 that would otherwise be emitted along with clean H2 to Celanese methanol plant |
| Start-up: | 2024 |
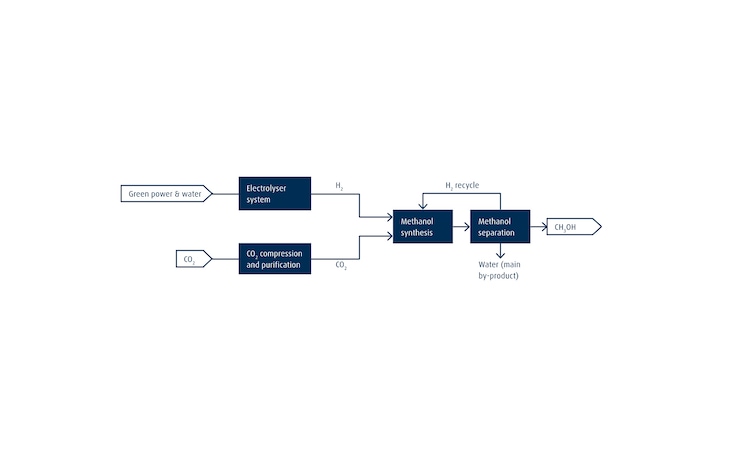
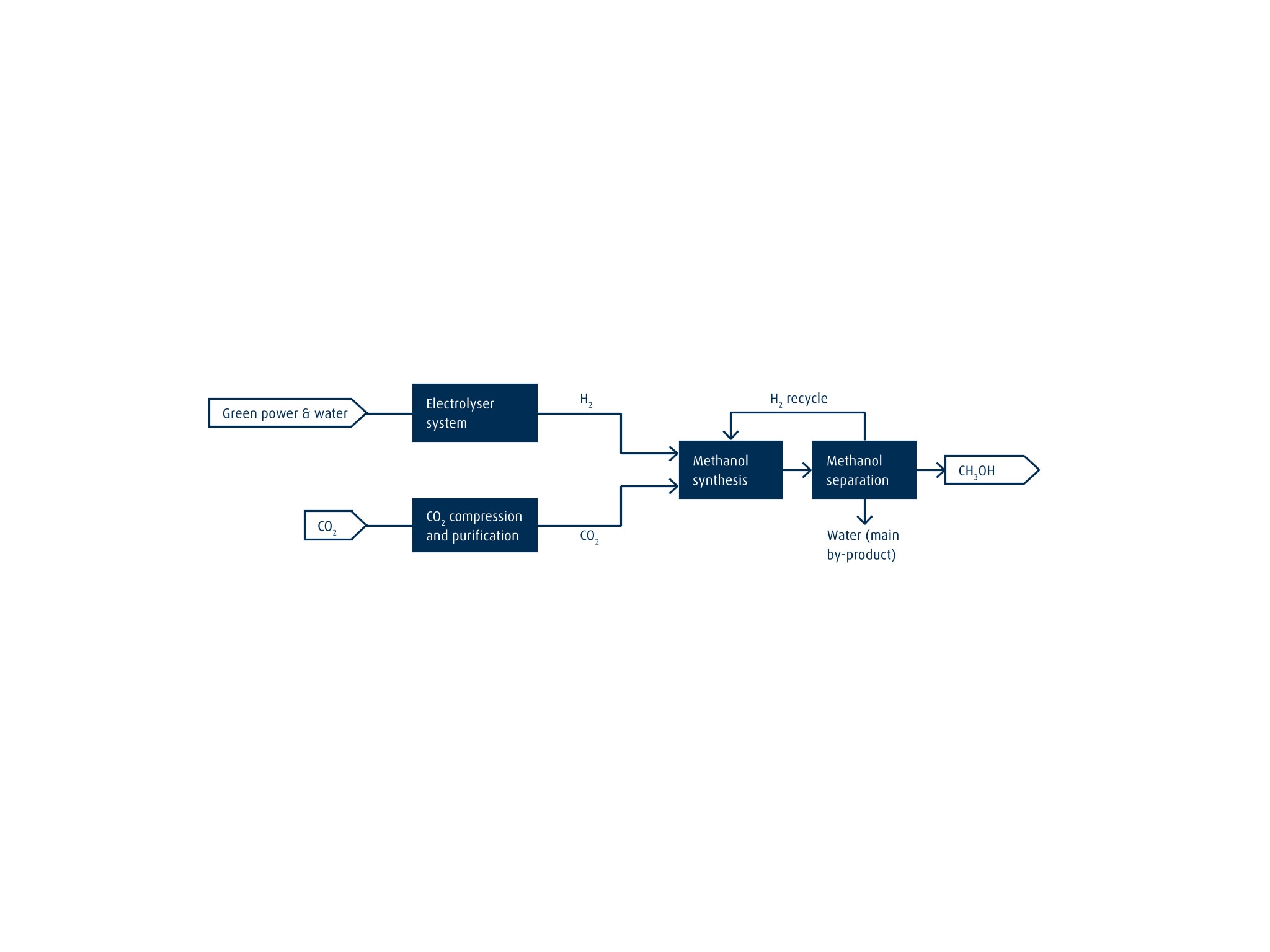
Putting Captured CO2 to Good Use: E-Methanol
Our offering includes plants for e-methanol production. As illustrated below, this involves using CO2 captured from off-gas streams from cement production, refining operations or similar industrial processes as a feedstock. It is thus an excellent showcase for a potential carbon capture and utilization (CCU) use case.
With e-methanol, the imported CO2 is mixed with green H2 produced by means of electrolysis to generate a H2/CO2 syngas mixture. The MeOH is synthesized in a Linde isothermal reactor. The process is similar to that used for conventional MeOH production although different catalysts are used. Replacing CO with CO2 in the feedstock changes the syngas composition and - by extension - the catalyst requirements. Process efficiency is the same in both cases, however.
Once the MeOH has been separated, H2 is recovered and recycled to the synthesis step for maximum process efficiency. To accommodate fluctuations in the availability of renewable power, we have developed flexible load concepts and dynamic control schemes (including highlights such as hot stand-by systems).
Greater Flexibility Through Hybrid Concepts
Drawing on the exceptional depth and breadth of our portfolio and proprietary technologies, we combine various process paths to create hybrid concepts tailored to individual needs, feedstock availability and the price of natural gas, electric power and CO2. Concrete examples include complementing conventional MeOH production technologies with renewable H2 from electrolysis to fine tune the H2 to CO/CO2 ratio for greater efficiency and lower carbon intensity.
Ready for the next step?
Talk to one of our experts and discover how you can reduce your carbon footprint with our engineering innovations - today.